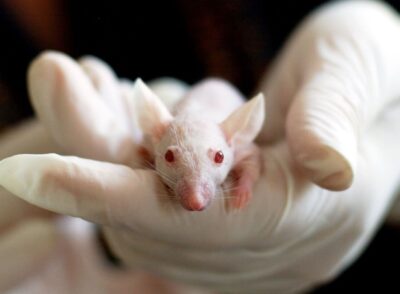
UNCOMMON
PRE-CLINICAL AND CLINICAL
CRO BASED IN INDIA

SOLUTIONS PARTNER
from Pre-clinical to clinical research

AND CLINICAL EXPERIENCE
GREAT TECHNOLOGY.
GREAT FLEXIBILITY

EXPERIENCED TEAM

QUALITY SERVICES
FOCUS ON CLIENTS

FLEXIBILITY
WE’RE HERE TO CHANGE THE WAY THE WORLD ENCOUNTER CRO'S, IN THE BEST POSSIBLE SOLUTIONS.
Krescent Medical Research is a full service pre-clinical and Clinical Research Organization (CRO) that supports pharmaceutical, biotechnology and medical device companies throughout their entire drug development journey. Also, as a collaborator it’s our duty to guide you through the complexities of the drug development and regulatory submission process. We impart strategic decision-making and operational support for your pre-clinical, clinical trials and regulatory submission projects by truly creative, innovative, and scientific thinkers.
We are a passionate drug development organization committed to improving medical research model by offering quality professional services with low overhead at justifiable, competitive industry pricing.
Krishnaa Upadhye
(PRESIDENT AND FOUNDER)
We try to serve best solutions
FAQ form Krescent
You will learn more from FAQ
WHAT IS KRESCENT MEDICAL RESEARCH?
HOW DO YOU SELECT SITE’S FOR CLINICAL TRIAL?
We already have created links and laces with the investigators we have worked with, that let us count on with their confidence to reach the success on every investigation we take part, being them our principal prescribers.
With this investigators database we could also help the sponsor to select the best sites all over India through our site selection system and database that ensure the feasibility of the services of the site (Pharmacy, Laboratory, Image Service, General Resources and Human Resources) according to the needs of the study.
HOW DO YOU DESIGN AND PLAN FOR CLINICAL TRIALS?
Necessary intelligence and creativity are applied on developing a global strategy which helps on saving resources to implant the medicine in the major markets possible, considering that a good global planning avoids duplicating efforts at the same time that simplifies the adaptation to the international standards to the specifics regulations of each country.
We share the knowledge of our experts on design effective programs that allows the adaptation to each country regulations and an optimal distribution of the medicinal devices and pharmacy products.
HOW DO YOU GET WORK DONE FROM REGULATORY AGENCY/EC?
We work and count on with and extend net of sites and their Ethics Committees, that allows us to warranty the minimum consumption of needed resources to get the EC approval in each project we became involved.
Necessary intelligence and creativity are applied on developing a global strategy which helps on saving resources to implant the medicine in the major markets possible, considering that a good global planning avoids duplicating efforts at the same time that simplifies the adaptation to the international standards to the specifics regulations of each country.
MEET THE PEOPLE WHO LEAD THE WAY TO A BETTER CLINICAL EXPERIENCE
At Krescent, our employees provide the support, structure, and professional background for better pre-clinical and clinical research.
Meet the people who lead the way to a better clinical experience.