WE’RE HERE TO CHANGE THE WAY THE WORLD ENCOUNTER CRO'S, IN THE BEST POSSIBLE SOLUTIONS
We are a passionate drug development organization committed to improving medical research model by offering quality professional services with low overhead at justifiable, competitive industry pricing.
Krishnaa Upadhye
(PRESIDENT AND FOUNDER)

WHY ALLY WITH KRESCENT?
We assist you overcome all the difficult challenges that arise during Pre-clinical and clinical drug development process.
There is no “one size fits all” approach to our policy. Our teams partner with you to understand your goals to plan and execute your clinical program to achieve success. And, we truly want to make a difference to help you get your medical therapy to market to improve or save people’s lives.
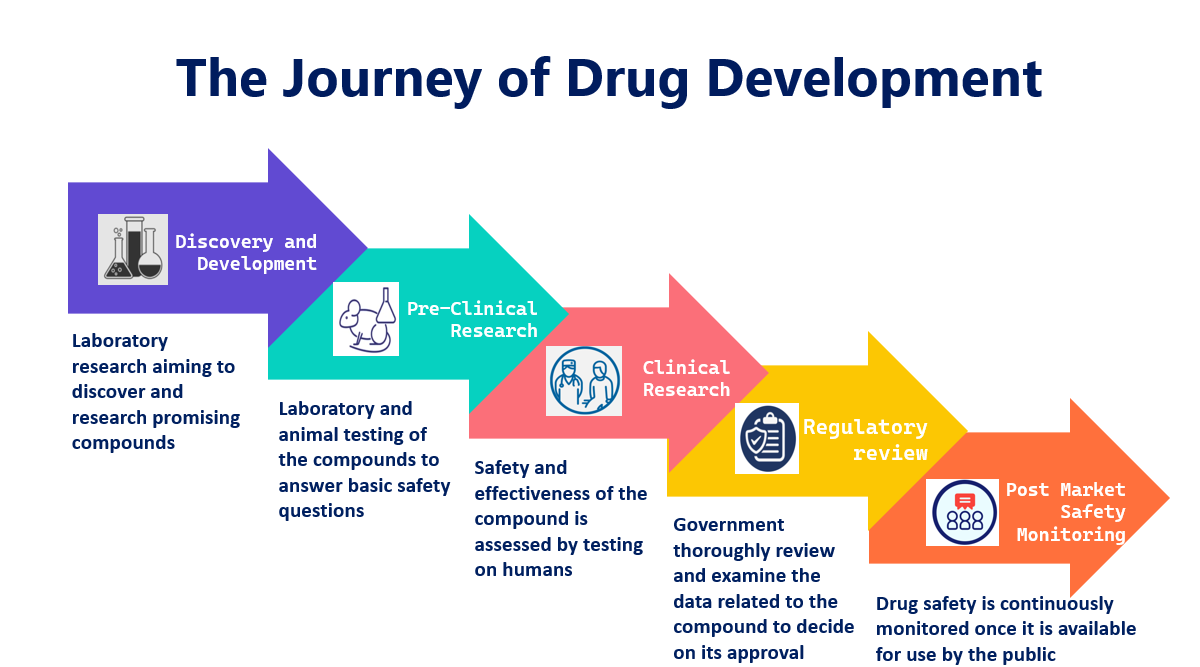
Navigating the Clinical Development Journey | Phase I-IV
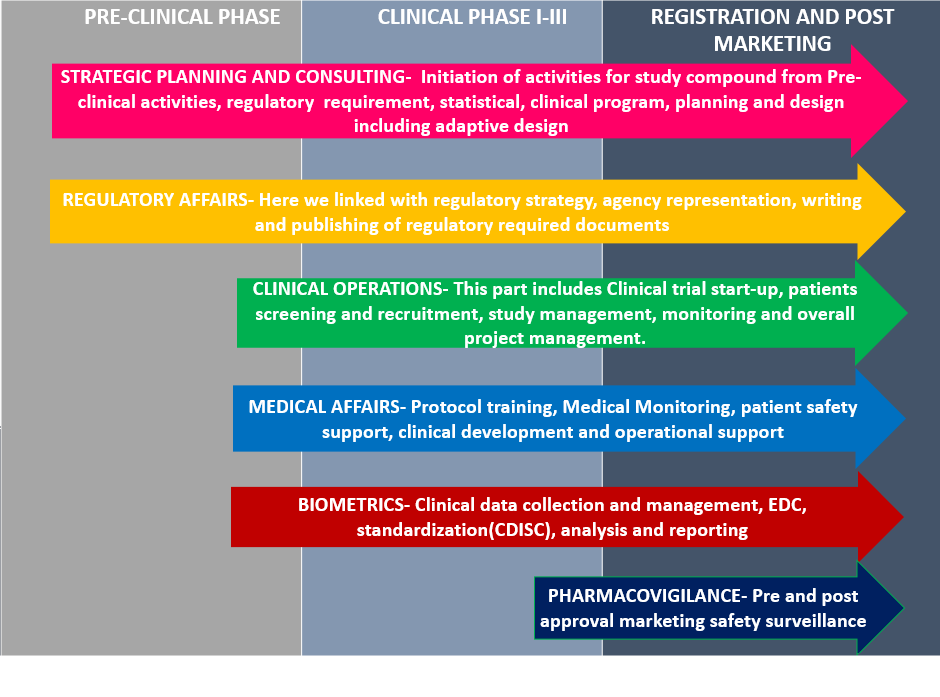
Benefits of Outsourcing?
Outsourcing to a Krescent Medical Research can bring multiple benefits to your organization.
- Time Savings
- Cost Savings
- Advanced Technological Needs
- Evolving and Complex Regulatory Requirements
A BETTER COMPANION
Whether you’re launching a pre-clinical study, clinical trial or seeking the next step in drug development programme, our pre-clinical, clinical research services help you achieve goals with greater confidence. We are a full-service CRO dedicated to enhancing the pharmaceutical and biotech experience. We believe in the power of collaboration. The best results are those built by a unified team. We provide extensive guidance and help mitigate challenges with the support of best-in-class tools, people, and partners.
At Krescent, we are willing to present exquisite data solution to the clients. Also, it is important offering risk management and meeting sponsor of high expectations, having dedicated teams and also sharing all crucial information with sponsors’ representative regarding to specific state/territory.
At Krescent, we look out the perfect study group in the perfect region of the India. If the data is not obtaining as expected, we change advancement. When something isn’t working we Invent, focus and execute. We do whatever it takes to perform our sponsor’s trial successfully. In compliance. On time, and on budget.
ALLYING WITH IMMINENT, SMALL & MID-SIZE BIOPHARMA COMPANIES.
We focus on providing effective strategies and impactful solutions to ensure the success of your pre-clinical and clinical studies. Regardless of your objective, we’ll be your GENUINE partner and lead you down the right path towards success.
Krescent is committed to building real Partnerships with our clients. For many of our clients, we literally become your project team, and for others, we provide the medical, scientific, clinical and statistical expertise to help you advance your therapy into and through clinical development.
At Krescent, building TRUE Partnerships with our clients is what makes us successful.
HOW WE STARTED;
CORPORATE HISTORY
Krescent was founded in year 2020 with a very simple goal: to provide pre-clinical and clinical consultancy and staffing support for India as well as oversea based pharmaceutical, Biotechnology, medical device companies. Our president and founder successfully enhanced our clients’ resources for pre-clinical and clinical research projects, and before long, an Indian MNC, 32-year-old, $100 M pharmaceutical company requested our services for innovative outsourcing solutions in clinical development. This event was the GENESIS of our first clinical CRO service offering.
Our personalized services and solutions, local regulatory expertise and therapeutic leadership are designed to face the most difficult industry challenges across all major therapeutic areas including oncology, gastroenterology, endocrinology, cardiology, nephrology and urology.
Our evolution continued, and today we are connecting to more global clients in over 5 countries.
Krescent provides full-service pre-clinical and clinical CRO, Functional Service Provider (FSP), Strategic Resourcing and Quality. Our employees average 08 years of experience in the pharmaceutical industry and are efficient at utilizing state-of-the-art tools and intelligence.
We strive to improve the way clinical research is performed and impact the future of health care using the most advanced technology and a challenge accepted approach.
MISSION FOR A CONCLUSIVE CHANGE
To provide high quality pre-clinical and clinical trial management services as a Contract Research Organization (CRO) to pharmaceutical, biotechnology and medical device companies.
To assist our partners in bringing new and innovative medicinal products to market in a timely and cost-effective manner
Krescent’s mission is to provide clients a better pre-clinical and clinical experience. We care about and respect the patients who make progress in clinical trial possible, and we’re committed to enhancing their quality of life while securing success for our pharmaceutical, biological, and medical device clients.
A VISION POWERED BY ALLIANCE
To become a reputed Contract Research Organization (CRO) of reference to pharmaceutical and biotechnology sponsors for the management of pre-clinical and clinical studies and the development of innovative treatments worldwide.
As your trusted partner in pre-clinical and clinical research, our vision is to provide value-based solutions through a high-performing, one-team approach and we are always passionate about our clients and their upcoming projects. It’s our responsibility is to contribute and explore to the ongoing global evolution of medicine; to reduce the burden of drug development timelines, and facilitate access to global medicine for global patients.
VALUES THAT ADVANCE BENEVOLENT PROGRESS
At Krescent, we ensure universal respect for our clients, while delivering CRO services nothing short of excellent. We hold ourselves accountable in everything we do, and we practice great leadership through every pre-clinical and clinical challenge and breakthrough.
CUSTOMER-FOCUSED
As a trustworthy partner, we focused on customer service, understanding the goals, expectations, care, respect the culture, accountability and leadership. Our relationships with customers are based on trust, commitment, and transparency.
EFFICIENCY
We seek to accomplish more by doing less, improving our productivity by working smarter.
PASSION
We do amazing things because our goals are fueled by passion
INNOVATIVE
We endeavor to keep our brains sharp by thinking differently, looking to solve problems in ways we have not seen before. We approach "crazy" with respect and a fresh perspective.
INTEGRITY
Integrity is at the core of our service. We seek to demonstrate a fully honest, truthful, and ethical conduct to our clients and partners at all levels.
RELIABILITY
Our services are reliable, professional, and proactive. We are a robust company both technically and financially.
QUALITY AND TRANSPARENCY
The quality management policy of Krescent is focused on our customers. We seek increased satisfaction related to the expectations and needs of our customers and other interested parties, by constant improvement of service quality and transparency
QUALITY POLICY AND SOCIAL RESPONSIBILITY
Quality is the cornerstone of Krescent Medical Research, Krescent has always held to the principles of running a continual business, standing by its set of values as well as retaining and contributing to the surroundings. Our goal is to establish lasting values and core principles, create excellent working conditions and give back to the community.
When our sponsors entrust their pre-clinical and clinical studies at Krescent, they can be confident that our start-up, regulatory, data management, clinical operation, medical writing and SMO services will be provided in accordance with the highest quality management principles and international standards.
Krescent recognizes the critical importance of Good Clinical Practice (GCP) and the need to guarantee patient rights and safety, as well as the reliability of data in clinical trials. The company views these aspects as a primary responsibility that governs its clinical operations, and has adopted appropriate quality standards to ensure study success and customer satisfaction.
Our team has a deep knowledge of pre-clinical and clinical trial legislation and wide experience in multiple therapeutic indication studies, to deliver the highest quality of service.
- compliance with applicable regulations,
- the client’s expectations and requirements regarding the services provided
- comply with all applicable legislations and standards
- train our staff in the needs and responsibilities of quality management
- ensure that our services meet the highest standards of quality and cost effectiveness
- follow a concept of continuous improvement and make best use of our management resources in all quality matters
- communicate our quality objectives and performance throughout the company and to interested parties
We don’t rest on our well-earned reputation for high-quality or relax our ideals. We treat every project as an opportunity to apply lessons learned and excel again. Relationship building with us is not about settling into a groove, but continuing to impress you and meet your needs.
We position our clients at the forefront of their field by advanced services.
Consultio is a professional consulting company
2nd Feb, 2018
Exhibition Planning & Exhibition Management21st Jul, 2018
Growth internationallyfirst half of the 2018s19th Aug, 2018
The purpose of the business plan2nd Jan, 2019
Focus business history on what matters to planning22nd Sep, 2019
History to Unite and Inspire People12th Jan, 2018
Establishment of Constrio8th Jul, 2018
Registered as a construction company18th Aug, 2018
Construction bought the Greek company Delta27th Sep, 2018
For lean business plans, operational plans, and strategic plans8th Jul, 2019
Award winner
We have many reviews from our satisfied clients.

Kathleen Smith

Van Hunter

Macquarie Telecom









